Post-mortem protocol for the Anaesthetic Death Investigation
This basic post-mortem examination should take 20-30 minutes. Most of the time is spent stitching the incision so the owners can have the body back for burial after the procedure.
The tissues that need to be collected for submission to Abbey Veterinary Services are:
- Heart and lungs (whole organs)
- Piece of liver*
- Whole kidney (sectioned)
- Whole or part of the spleen
* It is recommended that two additional unfixed samples pieces of liver are frozen in a sealed container and retained at the practice for PCR testing if RHD is diagnosed from histopathology and the owner or vet wishes to know whether it is RHDV1 or RHDV2.
The histopathologist is willing to look at additional tissues. If any other tissues of interest are collected during post-mortem examination, they can be included and sent with the rest of the samples.
Preparation
The time that the examination can be reduced by having all the necessary equipment to hand. This includes:
- Access to an X-ray machine if pre-operative radiographs are not available
- Protective clothing and disposable gloves
- Clippers (optional)
- A set of suitable instruments including scalpel blades, suture needles and suture material
- A large necked container (e.g. a clean tub that had tablets in it or a plastic lunchbox) to put the tissue samples in. Fill it with 50 to 100mls formol saline to fix the tissue.
- A camera close to hand (optional). Photos of abnormalities are always useful. A mobile phone protected by a plastic bag with a window cut out over the lens works well.
- A magnifying glass (optional).
Protocol for post-mortem examination
- Take a lateral radiograph of the chest and abdomen if pre-anaesthetic radiographs are not available.
- Clip the fur from the midline (optional). This makes the procedure easier because it prevents annoying hair from entering the body cavity and blood contaminating the fur. However, it lengthens the procedure time and also makes the incision more obvious to the owner.
- Examine the body for any external abnormalities. If possible, check the oral cavity for the presence of food material, blood, or pus. Were there any dental abnormalities?
- Make a midline skin incision from throat to pubis and expose the musculature beneath
- Free some of the skin from the musculature so that it is away from the incision. This also reduces the amount of hair that enters the body cavities.
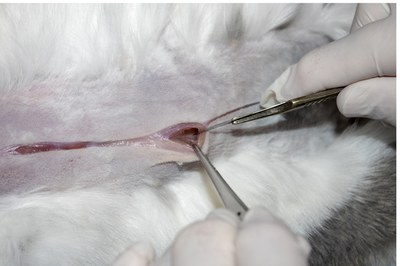
- To open the abdomen, tent the muscle layer and made a small nick along the midline so that air can enter and the linea alba can be cut without opening the caecum, which lies beneath.
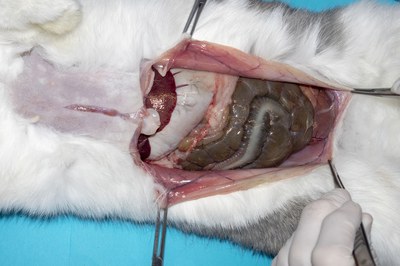
- Extend the incision through the linea alba. It can also be helpful to incise the muscles following the line of the ribs and the pubic brim. This allows the muscles to be folded back to expose more of the viscera without letting them displace too much but it takes a little longer to repair the incision at the end of the procedure.
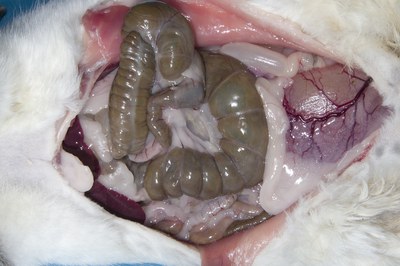
- Look at the gross appearance of the abdominal viscera for any obvious abnormalities such as tumours, abscesses, fluid, blood, or ingesta in the abdominal cavity. Is there evidence of gastric ulceration?
- Is the stomach distended and filled with fluid and gas? If so, look for a small intestinal obstruction especially at the pyloric end of the duodenum. The common causes of obstruction are pellets of impacted fur or neoplasia.
- Examine the liver and assess its size and colour. Take a representative sample (about 2x1x1cms of tissue) and place it in the formol saline for histopathology. If there is local pathology take a section from normal to abnormal tissue.
- Collect two more 2x2x1cm liver samples to freeze (unfixed) in case PCR testing for RHD is indicated from the histopathology results (optional).
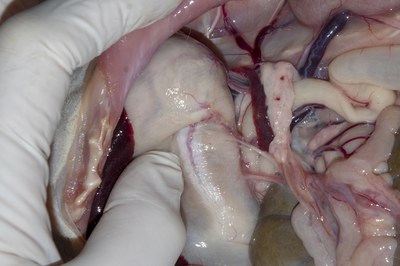
- Lift the stomach and examine the spleen attached to the stomach by the lesser omentum. Remove at least half the spleen and put it into the formol saline.
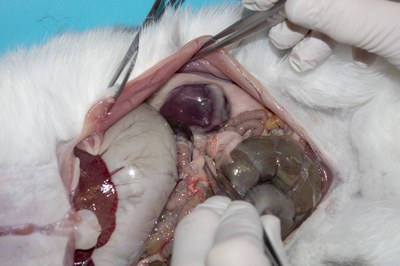
- Move the viscera to the right and find the left kidney (and adrenal gland). Note the size and surface of the kidney.
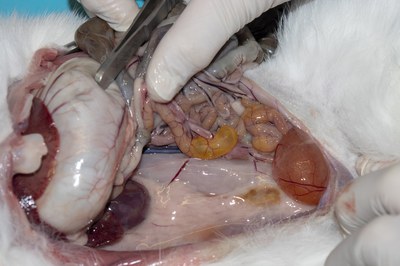
- Do the same on the other side and examine the right kidney and caudal process of caudate liver lobe.
- Remove at least one kidney and section it longitudinally before placing it in the formol saline.
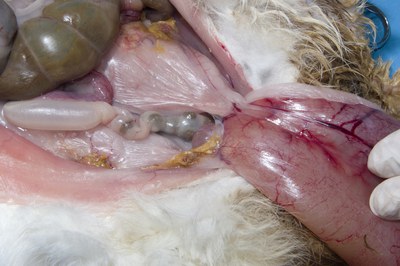
- Examine the lower urinary tract. If the rabbit is a neutered female, check for adhesions between the bladder and uterine/vaginal stump (illustrated).
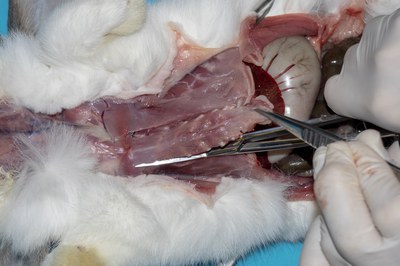
- Make a small nick in the diaphragm to allow the lungs to move away from the diaphragm and ventral thoracic wall. Incise along the costochondral junctions on either side of the sternum. The sternum can then be removed.
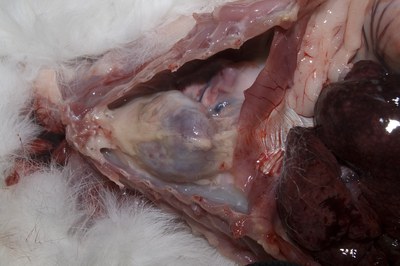
- Examine the thoracic cavity for the presence of fluid (shown), mediastinal masses or abscesses Note the external surface of the heart and pericardium. Look at all the lungs in situ.
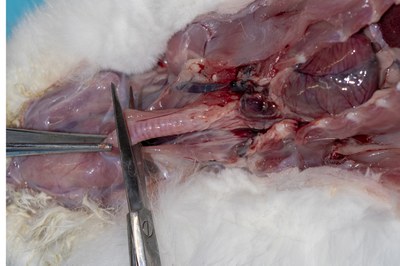
- Incise up the neck to the throat and expose the trachea. Section the trachea.
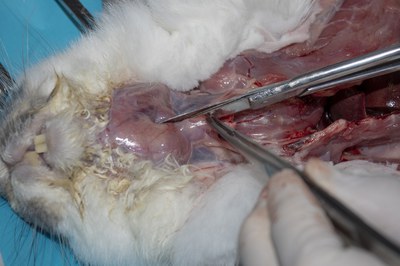
- Open the trachea to the pharynx to check for the presence of a foreign body. Note: The internal surface of the trachea in rabbits is naturally hyperaemic and red in colour.
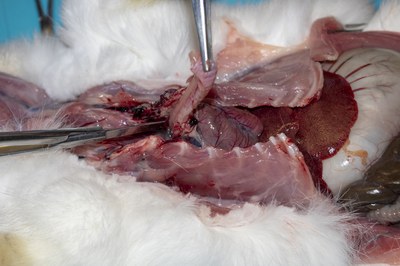
- Grasp the trachea and gently lift out the pluck (trachea, heart, and lungs) after freeing it from the diaphragm by sectioning the oesophagus, vena cavae and aorta.
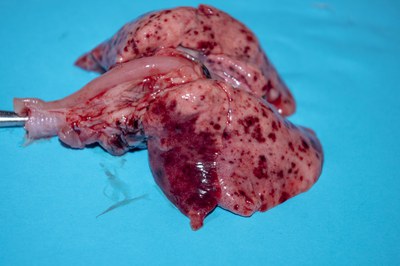
- Place pluck on a flat surface. Look at the heart and lungs. Does the heart look big? Are the lungs discoloured, collapsed and reddish/pink in patches (illustrated). Are there any obvious tumours, or abscesses?
- Lift the pluck and hold the cut edge of the trachea in forceps. Fix the lungs internally by trickling 5mls or more of formol saline into the trachea until the lungs are fully inflated.
- Incise the pericardium and inject approximately 2mls of formol saline into the chambers of the heart, especially if the heart is large.
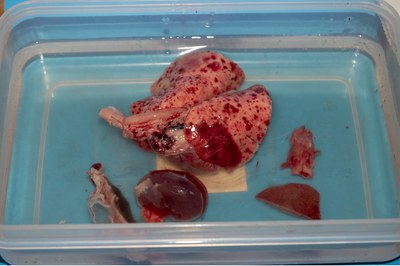
- Immerse the whole pluck with the other tissue samples in formol saline.
- Repair the incision. Paper towelling can be used to pack the chest cavity and absorb any blood
Additional tissues (optional)
The histopathologist is willing to look at any additional tissues that are submitted. If any abnormal tissue is found during the post-mortem examination, it can be included and sent with the rest of the samples.
Intestinal pathology
Because of autolysis, meaningful results can only be gained from intestinal tissue if it is really fresh i.e. within one hour of death. It is helpful to submit samples from different areas of the gastrointestinal tract, even if they look normal on macroscopic examination. The samples need to be labelled so the histopathologist knows which parts the samples came from.
For best preservation, isolate the affected area of gut by tying off the whole piece of bowel. This allows the lumen of the gut to be injected with formol saline. Distend the bowel so that it keeps its form but is not under pressure.

Remove and place in formol saline. When the samples have fixed, they can be placed in individual plastic bags labelled with the section of the intestinal tract that the samples came from.
Alternatively, open a length of the intestine and place it with the peritoneal aspect onto a wooden tongue depressor before placing it in formol saline. The part of the intestine that the sample came from can be written in pencil on the wood prior to fixing the tissue onto it.
CNS tissue.
Collection of CNS tissue is time-consuming, and the body cannot be returned to the owner at the end of the post-mortem examination. They need to be informed of the deconstructive nature of the examination.
The histopathologist needs the whole brain and/or a length of cord to work with. It is unusual for random pieces of brain to be useful so the histopathologist needs to know which part of the brain they are looking at. The brain needs to retain its anatomical form and to be properly fixed, which makes it easier to remove. There are two ways to do this after the skin is removed from the skull to expose the bone.
- Carefully remove the dorsal and caudal parts of the cranium before filling the cranial cavity with formol saline and allowing the brain to fix before it is removed.
- Carefully remove the dorsal and caudal parts of the cranium before sectioning the brain longitudinally through the sulcus and filling the cranium with formol saline. This allows the fixative to penetrate into deeper tissue. The whole head (after removing the skin), with the brain in situ, can then be immersed in formol saline before submission to the laboratory. This allows the pathologist to orientate the tissue.
Fixing and posting the tissue samples.
Once the other tissues have fixed (more than 48 hours), the formol saline can be drained off and the fixed tissues placed in a sealable plastic bag.

This bag is then surrounded by paper towels, placed into another sealable bag and finally a padded envelope. This provides sufficient protection for the tissues without posting liquid formol saline. Enclose the submission form that will be generated from our website after entering the details of the case and submit the samples to Abbey Veterinary Services
